Wes国内最新成果!医科院病原所金奇团队结核杆菌基础研究、诊断标志物筛选、疫苗开发
前言
自结核杆菌发现至今,已有近140年历史,但结核病仍然是对全球健康的重大威胁,是导致死亡的主要原因,特别是在发展中国家。由于结核病全球死亡率与艾滋病并列第一,大多数国家都在响应世卫组织“2035年结束结核病战略”。
作为一个结核病负担高的国家,中国开发了新的工具,特别是在结核病诊断方面,以减少结核病的流行。目前方法并不优于结核菌素皮肤试验,也不能区分潜在结核杆菌感染和活动性结核且漏诊率较高,仅60%~80%的活动性肺结核可采用现有的诊断方法检出。
因此,识别新的宿主和病原体的生物标志物是提高结核病诊断准确性的关键。最近,科学家们正致力于破译每一种结核杆菌基因或蛋白质功能。在4,000多个结核杆菌开放阅读框架(ORF)中,分泌蛋白(如ESAT-6、CFP-10和Ag85A/B)被认为能刺激抗原特异性免疫应答,并被广泛用于诊断和疫苗研制。然而,这些蛋白质的使用仍然受到限制。
临床上,因为它们不能用于结核病感染和结核病的鉴别诊断,只有70%的阳性临床结核病患者被这些蛋白所识别。医科院病原所金奇实验室已对1250种蛋白质(约占结核杆菌蛋白的三分之一)的功能进行了全面的研究。
结核杆菌膜蛋白抗原性研究
医科院病原所最近发表文章:Analysis of the Antigenic Properties of Membrane Proteins of Mycobacterium tuberculosis,(2019) 9:3042 | https://doi.org/10.1038/s41598-019-39402-z 1,集中研究结核杆菌膜蛋白。结合杆菌膜蛋白一直被认为是免疫原,是指与生物膜相互作用或构成生物膜的蛋白质,包括永久锚定或构成膜的完整膜蛋白,以及仅暂时附着在脂质双层或其他整体蛋白质上的外周膜蛋白。
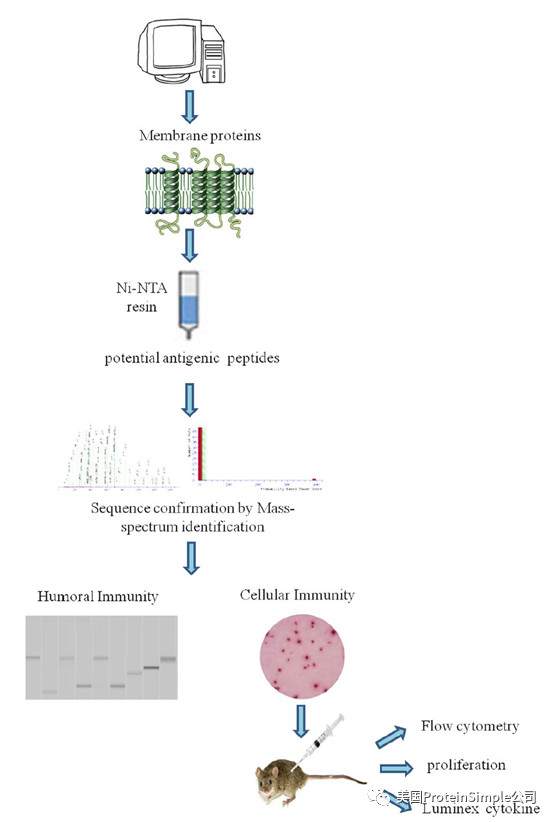
为了更好地了解这些蛋白,科学家表达并纯化了所有结核杆菌膜蛋白,并通过三轮血清学免疫检测来确定其作为潜在血清学诊断标志物的价值。此外,还进行了两轮细胞免疫试验,以评价这些蛋白质作为筛选生物标记物的适用性。进一步分析结核病患者细胞抗原反应所产生的膜蛋白抗原,可加速抗原生物标志物的研究,提高结核病诊断和疫苗的研制水平。
研究路线图
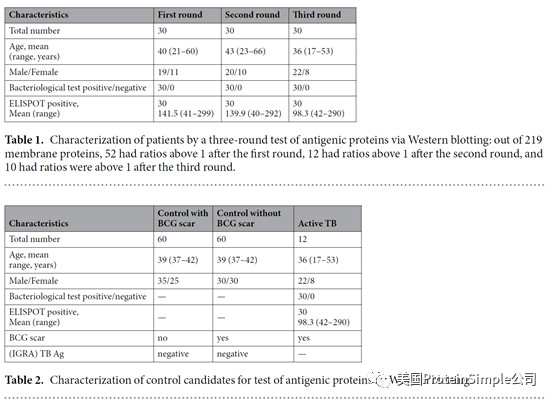
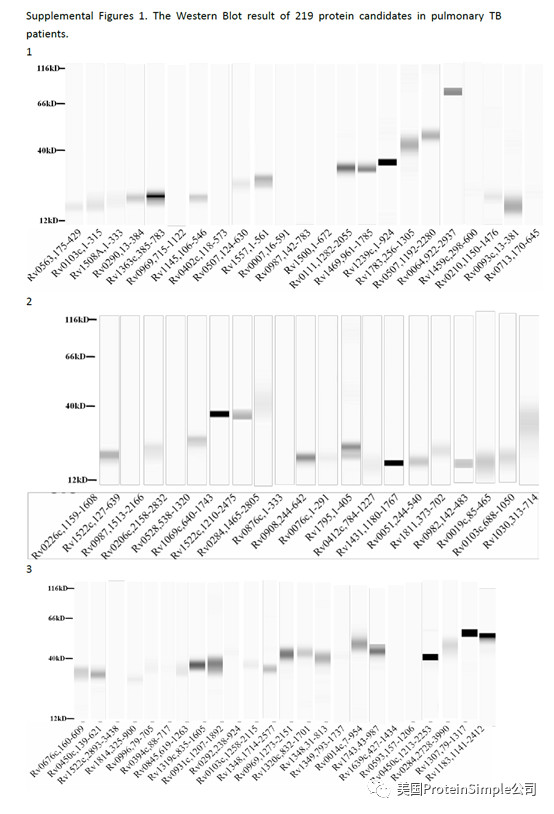
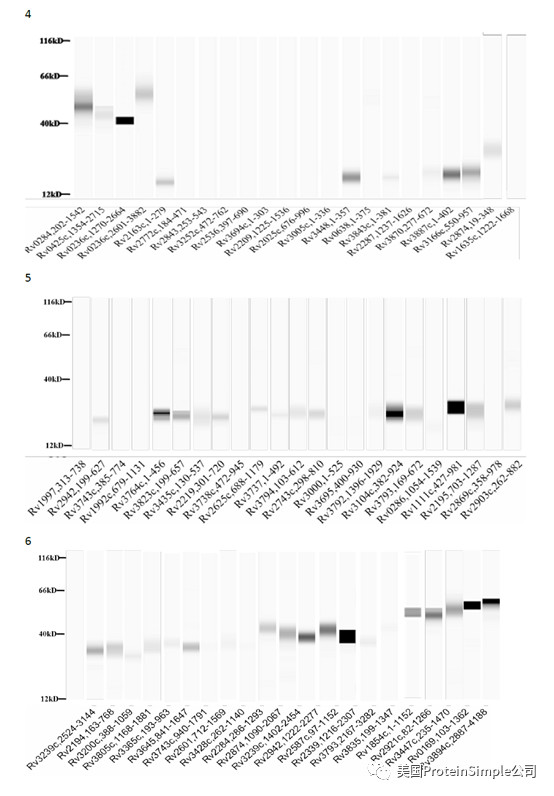
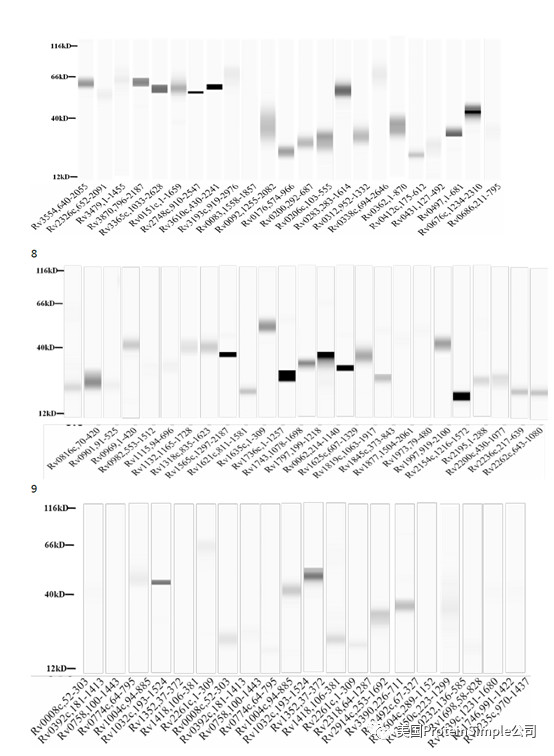
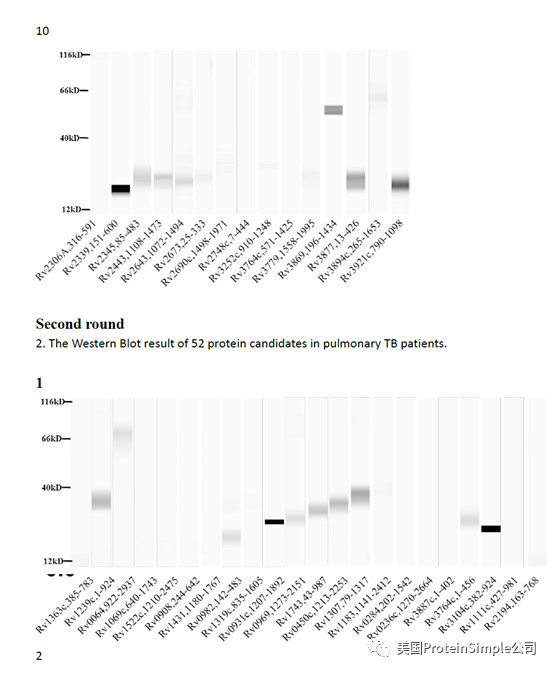
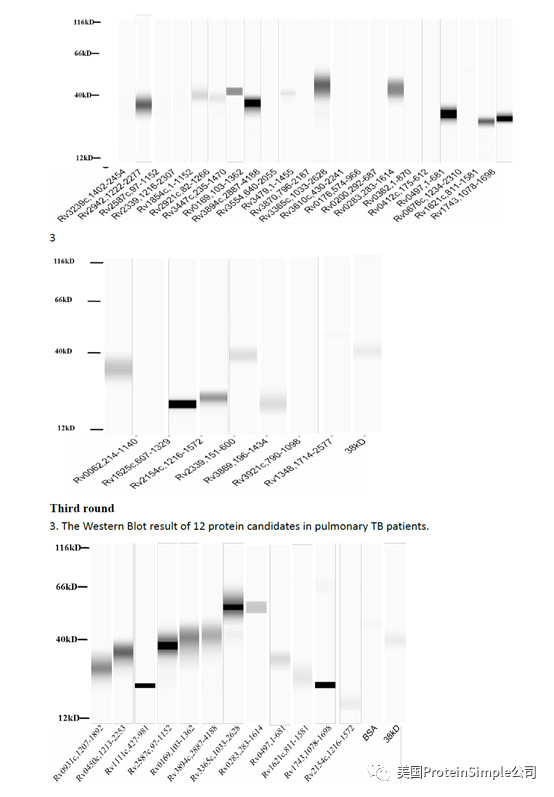
Wes全自动定量western blot系统承担了本研究最重要的血清抗原分析(219种结核杆菌膜蛋白病人血清结合实验)
Identification of M. tb serological antigens by Western blotting.
Although humoural immunity remains an auxiliary means of diagnosing TB, we expected the results of Western blotting to provide us with useful M.tb-specific 6 antigens for serum IgG, as based on our previous method. Transmembrane proteins play important roles in the transport of substances and immune protection. To assess the antigenic properties of the induced humoral response in patients, Western Blotting was performed to identify serological antigens in pulmonary TB patients (Table 1) and healthy controls (Table 2). To avoid the influence of Bacillus Clamette- Guérin (BCG) vaccination, the healthy controls included individuals with or without a BCG vaccine scar. Additionally, a positive protein response to active pulmonary TB patient serum was used to screen control candidate serum (data not show). All serum samples were pretreated to remove the E. coli background as reported previously and bovine albumin (BSA) and commercial Rv0934 were used as the negative and positive control. To evaluate the antigenicity of each M.tb protein, the serum response intensity ratio of expression level of the target proteins to that of Rv0934 was calculated; if the ratio was equal to or greater than 1, the protein was confirmed in second and third rounds. After the first round of testing, we obtained positive responses for 161 of the 219 membrane protein candidates (approximately 74%). After the second round, 52 membrane proteins had a ratio greater than that of Rv0934 (Fig. 2, Supplemental Tables S1, Figs S2–S4); the yellow bars indicate positive proteins, and the ratio ranged from 0.02% to 338%. After three rounds, 18 of the remaining membrane proteins had ratios above 1, (Fig. 2, dark blue bars). The ratio of Rv1111c (427–981) was particularly high (a mean ratio at 3.38).